3.6V AA ER14505 Li-SoCl2 Battery (2400mAh) | Pkcell
Understanding ER14505 Battery
The ER14505 battery is a 3.6v lithium battery. It mainly uses lithium thionyl chloride (Li-SOCl2) as its chemical. This battery is a primary type. This means it can not be charged and is for single use only. Like an AA battery, it measures 14mm wide and 50.5mm tall. The ER14505 battery 3.6V gives a higher voltage than standard alkaline AA batteries. It also has a lower self-discharge rate. These features make 14505 battery ideal for devices that need steady and long-lasting power. This includes remote monitoring systems and utility meters that need a reliable power source.
Key Features of Pkcell ER14505
- High and Stable Voltage
- High Minimum Voltage During Pulsing
- Stainless Steel Container
- Hermetic Glass-to-metal Sealing
- Low Self-discharge Rate (Less than 1% After 1 year of Storage at +25℃)
- Wide Operating Temperature (-60℃~+85℃)
- Restricted for transport(class 9)
Main Applications of Pkcell ER14505
- Gas Metering
- Utility Metering
- Alarms and Security Devices
- Memory Back-up
- Tracking Systems
- Professional Electronics
Cost-effective Alternatives to Tadiran, Saft, Xeno & Tekcell
| PKCELL ER Model | Equivalent Competitor Model(s) |
|---|---|
| ER26500 | LS26500 (Saft) |
| ER14505 | LS14500 (Saft), XL-145F (Xeno), Tekcell SB-AA02, TL-5186 (varies) |
| ER14250 | TL-5902 (Tadiran), Tekcell 1/2AA, TL-2150, XL-060F, XL-055F, SL-760, TL-5104, LS14250 |
| ER17505 | LS17500 (Saft) |
| ER34615 | LS33600 (Saft), TL-5930 (Tadiran), TL-5920, XL-205F, LSH20 (Saft) |
| ER18505 | LS-18505, TL-5955 (Tadiran) |
| ER17335 | LS-17335, TL-5903 (Tadiran), TL-5903S |
Specification Of Other Li-SOCl2(Energy Type) Models
| Model IEC | Nominal Voltage | Dimensions | Nominal Capacity | Standard Current | Max Continuous Discharge Current | Max Pulse Discharge Current | Cut-off Voltage | Weight Approx | Operating Temperature | |
|---|---|---|---|---|---|---|---|---|---|---|
| ER10450 | AAA | 3.6 | 10.0×45.0 | 800 | 1.00 | 50 | 60 | 2.00 | 9 | -55~+85 |
| ER14250 | 1/2AA | 3.6 | 14.5×25.0 | 1200 | 0.50 | 20 | 45 | 2.00 | 10 | -55~+85 |
| ER14335 | 2/3AA | 3.6 | 14.5×33.5 | 1650 | 0.70 | 75 | 150 | 2.00 | 13 | -55~+85 |
| ER14505 | AA | 3.6 | 14.5×50.5 | 2400 | 1.00 | 100 | 200 | 2.00 | 19 | -55~+85 |
| ER17335 | 3.6 | 17×33.5 | 2100 | 1.00 | 100 | 200 | 2.00 | 30 | -55~+85 | |
| ER17505 | 3.6 | 17×50.5 | 3400 | 1.00 | 100 | 200 | 2.00 | 32 | -55~+85 | |
| ER18505 | A | 3.6 | 18.5×50.5 | 4000 | 1.00 | 120 | 200 | 2.00 | 32 | -55~+85 |
| ER26500 | C | 3.6 | 26.2×50.5 | 8500 | 2.00 | 130 | 300 | 2.00 | 55 | -55~+85 |
| ER34615 | D | 3.6 | 34.2×61.5 | 19000 | 3.00 | 200 | 400 | 2.00 | 107 | -55~+85 |
| ER9V | 3.6 | 48.8×17.8×7.5 | 8500 | 2.00 | 130 | 300 | 2.00 | 16 | -55~+85 | |
More Than Just Individual Cells - We Offer Complete Power Solutions.
- Single Battery With Cables and Connectors
- Custom LiSOCl2 Battery Packs Based on Your Tailored Needs
Why Choose Pkcell Battery?
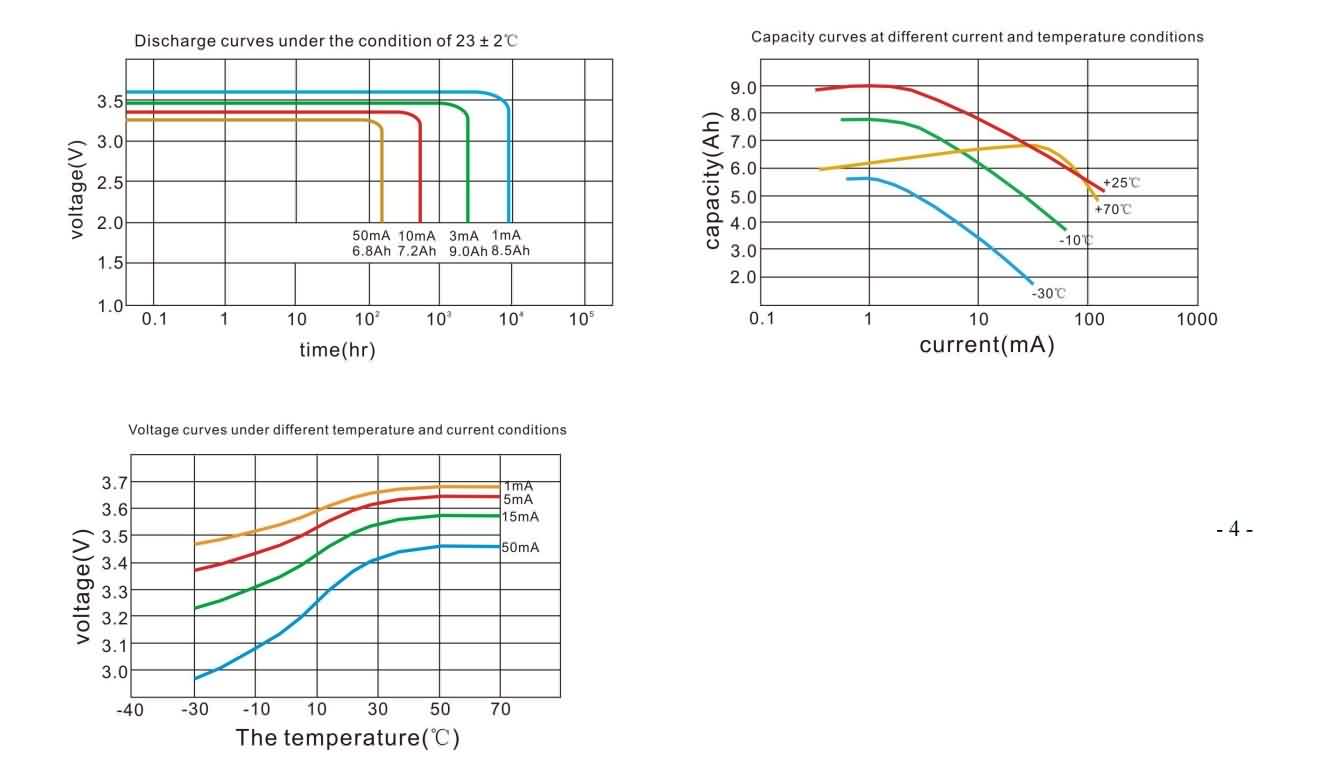
Typical Discharge Characteristics
Warning:
- These are non rechargeable batteries.
- Do not recharge, short circuit,crush, disassemble, heat above 100℃ incinerate.
- Do not use the battery beyond the permitted temperature range.
Frequently Asked About LiSoCl2 Battery
- Lead Time: Standard samples typically arrive in 7-12 days. Formal orders usually take around 25 days, though smaller quantities might ship faster, potentially within 15-18 days.
- Payment Methods: We accept various payment types, including T/T (Telegraphic Transfer), L/C (Letter of Credit), and PayPal.
- Shipping: We offer flexibility! Depending on your needs and location, we can ship your order via air freight (using carriers like FEDEX, DHL, UPS, EMS, etc.) or via sea freight.
- Delivery Terms: We can work with several common international shipping terms, including EXW, FCA, FOB, CFR, and DDU.
We have a minimum order value of USD $500. The actual quantity you receive depends on the unit price of the specific batteries you choose. Absolutely! We understand you need to test our products. We're happy to provide samples for your evaluation before you place a formal order.
Passivation is an interesting natural phenomenon observed in Lithium Thionyl Chloride (LiSO₂Cl₂) batteries! When lithium metal touches the thionyl chloride (SOCl₂) electrolyte, a thin, protective layer forms on the surface of the lithium negative electrode. This layer, mostly made up of Lithium Chloride (LiCl), creates a high-resistance barrier that prevents a continuous reaction between the lithium and the electrolyte. Isn't it fascinating how this process helps maintain the battery's performance?
Passivation has some excellent benefits along with a few potential drawbacks that are important to consider:
Benefits:
- Low Self-Discharge: The passivation layer does a fantastic job of slowing down any unwanted side reactions between the lithium and the electrolyte, leading to an impressively low self-discharge rate. Thanks to this, LiSO₂Cl₂ batteries can be stored for many years, often over 10 years, while still keeping most of their capacity intact.
- Long Shelf Life: Because of the low self-discharge, these batteries can hold onto their energy for quite an extended period, which is great for long-term storage.
Potential Drawbacks:
- Voltage Delay: When a passivated battery starts discharging—especially after being stored or during high-current pulses—the current first needs to break through or dissolve the high-resistance passivation layer. This can temporarily drop the battery's voltage below its normal operating level before it bounces back. This occurrence is often referred to as "voltage delay."
Passivation happens naturally during storage. To effectively break the passivation layer and reduce voltage delay:
- Apply a continuous load (discharge) to the battery. This current flow helps disrupt or dissolve the LiCl layer.
- The necessary load duration and magnitude to break the passivation can vary based on several factors, including the battery's size, how long it's been stored, the storage temperature, and the extent of passivation. Typically, using higher currents and allowing for longer discharge times are more effective in breaking down the layer. Once this layer is disrupted, the voltage will rise to the battery's nominal operating plateau.
Not at all! That initial lower voltage is usually just the battery "waking up." It's a direct result of that protective "skin" we talked about (passivation). The battery needs a moment to push through this layer, and then the voltage will rise to its normal level. It's a sign of a healthy, long-lasting battery!
It varies! It depends on how long the battery was stored, the temperature it was stored at, and how much power your device is drawing. Usually, it's very short, just a few seconds or minutes under a continuous load. For low-power applications, it might take a bit longer to fully recover.
While you can't prevent the passivation layer from forming during storage (it's what gives the battery its long life!), you can help "break through" it. Applying a continuous load to the battery for a short period is the most common way to activate it and bring the voltage up. The required load depends on the battery and application.
Yes, absolutely. Longer storage times and higher storage temperatures can sometimes lead to a thicker passivation layer, potentially causing a slightly more pronounced voltage delay when you first use the battery. Storing them correctly helps manage this.














